IMPORTANT NOTE: To open a linked file, don't just click on the file. Instead, do a right click, and choose "Open Link in New Window." If you don't do a right click, the file may not open properly.
IMPORTANT NOTE: To open a linked file, don't just click on the file. Instead, do a right click, and choose "Open Link in New Window." If you don't do a right click, the file may not open properly.
IMPORTANT NOTE: To open a linked file, don't just click on the file. Instead, do a right click, and choose "Open Link in New Window." If you don't do a right click, the file may not open properly.
IMPORTANT NOTE: To open a linked file, don't just click on the file. Instead, do a right click, and choose "Open Link in New Window." If you don't do a right click, the file may not open properly.
In the Christian religion it is believed that God first spoke to our first parents, Adam and Eve. When our first parents sinned, God promised them a Redeemer. Out of their descendants God selected certain individuals through whom He revealed Himself and His plan of salvation. He then selected Abraham to be the Father of His chosen people. It was from his lineage that a Redeemer was to come and save the world from its sins. Through the ages God continued to reveal Himself through the patriarchs and the prophets in preparation for the coming of the promised Redeemer: Jesus Christ, the Son of God.
The revelations delivered to the patriarchs and the prophets were therefore only partial revelations given to pave the way for Christ our Savior. It was through Christ that God’s full and final revelation was to come. In his letter to the Hebrews St. Paul said: “God, who, at sundry times and in divers manners, spoke in times past to the fathers by the prophets, last of all, in these days hath spoken to us by his Son, whom he hath appointed heir of all things, by whom also he made the world” (Heb. 1:1-2). Christ is, therefore, the Light of the World and the bearer of God’s full revelation.

A personal website of Mr. Romeo Maria del Santo Niño, OP
May 6, 2025 Edition

THE ORIGIN OF LIFE
Life on Earth
This essay is about the origin of life—not the life of angels or of God, but the life of living organisms on Earth. This is important because living matter is vastly different from lifeless matter, and life—or the vital processes in a living organism—are not reducible to mere physical and chemical processes. The origin of these vital activities in organisms is what we seek to find.
Some people have the mistaken idea that living bodies are nothing but sophisticated machines. Of course, machines also have different parts that work together, but every living body has parts that function harmoniously to serve the well-being of the whole body. The parts of the human body, for example, do not behave mechanically but coordinate to serve the needs of the whole body. Thus, in the presence of danger, certain processes in the body are accelerated. The bronchioles of the lungs are dilated to furnish a larger supply of oxygen; adrenalin and the secretions of the sweat glands are noticeably increased; sugar is released from the liver and discharged into the bloodstream for the production of more energy; and everything works to protect the whole body. Where did such vital activities come from? They are nowhere to be found in any advanced robot.
Also, every living body grows and reproduces itself. Lifeless matter may increase in size, but it does not really grow. Crystals might give the appearance of growth by the accretion of free crystals of the same kind into themselves, but not by assimilating foreign materials and changing them into their own substance. In contrast, a living organism, such as a fetus, may start as a cell or group of cells but quickly multiply to form organs so varied yet so coordinated as to constitute a fully developed organism of a certain kind. Moreover, a living organism reproduces itself in a way that lifeless matter does not do. The so-called “self-replicating machines” do make copies of themselves using raw materials outside their bodies. But it is nowhere close to the way living cells reproduce other living cells of their kind from within their bodies: by cell division. Where did this power come from?
The Question of the Origin of Life
It seems obvious that if living organisms did not just come out of nothing, then they must have originated from lifeless matter itself. Therefore, the main issue regarding the origin of life on Earth is not whether living beings originated from lifeless matter or not. The real question is: how? Did life on Earth originate from lifeless matter purely by chance? Or was there a hidden cause that directed its emergence from lifeless matter? The panspermia hypothesis, which holds that life on Earth originated from microorganisms from distant planets traveling through space and finding their way to Earth, will not be discussed here because it simply shifts the problem of life's origin to the other planet. The basic question remains the same: Did life on Earth originate from lifeless matter purely by chance?
The short answer is “No.” If chance alone were at play, then the likelihood of life occurring on Earth, or anywhere else in the universe, is extremely small. The only reason that there is life on Earth is that there had been a series of “lucky accidents” that happened in the past, from the beginning of the Big Bang until the present, that would have nullified the possibility of life if such accidents did not happen. A fluke, a lucky event, or an accident, even if highly unlikely, could happen by chance. But a fluke happening after a fluke, after another fluke, and so on, is a practical impossibility.
The Lucky Universe
That there is life in the universe is due to two lucky accidents. First, there was just enough matter at the beginning of the Big Bang to allow the universe to continue its course of producing galaxies, stars, and planets. Second, the magnitude of the four fundamental forces in nature – the strong nuclear force, the weak nuclear force, the electromagnetic force, and the gravitational force – is just right to enable the production of the elements necessary for life. Here are the facts:
-
If there had been too little matter in the universe at the beginning of the Big Bang, then the Big Bang explosion would have been so violent and the expansion rate of the universe so high that no galaxies would have formed. Without galaxies, there would be no stars. And without stars, no planets would form to serve as a habitat for life. If there had been too much matter at the beginning of the Big Bang, then it would have halted the expansion and caused the universe to collapse upon itself, again making life impossible. It was therefore a lucky accident that the amount of matter at the beginning of the universe was “just right” for the universe to expand at just the right rate to continue its evolutionary course.
-
Change the strength of the strong or the weak nuclear interaction within the atoms of matter and the result could be disastrous for life. If the strong nuclear force were too large, no hydrogen would form. If the strong nuclear force were too small, no elements other than hydrogen would form. If the weak nuclear force were too large, too much hydrogen would have converted to helium during the Big Bang and too many heavy elements would be produced by burning stars. If the weak nuclear force were too small, too little helium would be produced from the Big Bang, and atoms heavier than hydrogen would not be produced by burning stars. Again, it was just a lucky accident that the magnitude of the strong and the weak nuclear forces was “just right” to allow the formation of hydrogen and other heavier elements necessary for life.
-
The same is true of the electromagnetic force, or the force between electrically charged particles. If it were too high, the atoms would tend to keep their electrons and no sharing of electrons with other atoms would take place. If it were too low, atoms won’t be able to keep their electrons at all, and the sharing of electrons, which is needed for the formation of large molecules essential for life, would not exist. The gravitational force, or the force of attraction between masses, is also critical for life. If it were too strong, the stars would burn too quickly. If the gravitational force were too weak, stars would not be hot enough to produce atoms heavier than hydrogen and helium. It, therefore, seems that all the four fundamental forces of nature – the strong nuclear force, the weak nuclear force, the electromagnetic force, and the gravitational force – are, by some strange luck, precisely “calibrated” or “fine-tuned” to produce the elements necessary for life.
The Lucky Planet Earth

The Earth and Moon
Image source link: marysrosaries.com
The Planet Earth itself exhibits an amazing set of “lucky accidents” that make it stunningly perfect as a habitat for life. Here is a list of some of the Earth’s lucky accidents:
-
The solar system in which Planet Earth is located is neither too close nor too far from the center of the spiral Milky Way Galaxy. In other words, the Earth happens to be located in the habitable zone (also called the “Goldilocks zone”) of our galaxy. If our solar system had been closer, any living organism on Earth would have been exposed to deadly radiation from nearby supernovae and would not have survived; if it had been farther away, the elements required for life would have been in short supply.
-
The size of our sun, and the kind of star it is, is also “just right” to provide a fairly steady stream of energy needed to sustain life on Earth. If the sun were too small, it would emit too little light; if it were too big, it would emit too much heat.
-
The distance of the Earth from the sun is approximately 93 million miles, or 150 million km, which is “just right” for life. Had the Earth been closer, life would fry from the heat; had it been farther, all life would freeze from the cold. Even the Earth’s near-circular orbit is an absolutely lucky accident, for it ensures nearly the same distance from the sun all year round.
-
The Earth’s size is once again “just right” for life to exist on the planet. Had the Earth been too big, gravity would be so strong and everything would seem so heavy, making it difficult for fish, birds, and other large animals to move; had the Earth been too small, its gravity would be so weak that it would not be able to keep the atmosphere and its life-friendly gases close to the Earth.
-
The moon’s size is also “just right” for life to thrive on Earth. Had it been larger than it is, the ocean tides would cause disruptions to the Earth’s ecology; had it been smaller, the transfer of essential nutrients from the sea to the continents, and from the continents to the sea, would be insufficient.
-
The Earth’s tilt and spin are “just right” for life. Had the axial tilt been slightly different from 23.4 degrees, our beneficent cycle of seasons would have changed dramatically. Had the Earth’s spin been faster, the rapid rotation of the Earth would have generated turbulent cyclones, causing violent death and destruction. Had the spin been slower, the days would have been longer and the side facing the sun would have broiled, while the other side would have frozen.
-
The Earth’s magnetic field is another gift of sheer luck without which the Earth would remain unprotected from cosmic radiation and other lethal forces (solar winds, solar flares, explosions) from the sun.
-
The size of the Earth’s sea is another marvel of the planet Earth. The sea covers about 4/5th of the Earth’s surface, which is “just right” for it to support the natural water cycle that distributes fresh rainwater around the world. It is also the habitat of one-celled plants and seaweed that provide a store of water. Considering that the universe is full of hydrogen but little water, the planet is very, very lucky indeed for its huge store of water.
-
The Earth’s atmosphere contains just the right kinds of gases—oxygen, nitrogen, and carbon dioxide—without which the protein molecules and the other building blocks of life would not form. At the same time, the atmosphere is free of such poisonous gases as hydrogen, methane, and ammonia. What luck! In our atmosphere is an ozone layer that allows heat and light to come in but blocks the incoming ultraviolet radiation that is harmful to life. The atmosphere also provides a shield against the tons of debris that regularly come from space. Thanks to the atmosphere, this debris burns and reduces in size before it falls to Earth. Fortunately, the Earth has two giant neighbors, Jupiter and Saturn, which attract by gravity many asteroids and comets that would otherwise strike the Earth and annihilate many species.
-
The Earth’s soil mineralization is also important for life. If it were either too poor or too rich in nutrients, the diversity and complexity of species would be limited.
-
If the average surface temperature of the Earth rose just a few degrees, water vapor and carbon dioxide would accumulate in the atmosphere, causing the surface temperature to rise even more, resulting in a runaway greenhouse effect. Ice and snow would form on the Earth’s surface if the mean surface temperature were to fall by just a few degrees. Since ice and snow reflect solar energy better than uncovered land mass, the increased albedo (or the ratio of light reflected by a surface to the total amount of light falling on a surface) would lower the surface temperature further, causing runaway glaciation.
-
The natural water cycle of the Earth, the nitrogen cycle, the oxygen and carbon cycles, and indeed the entire ecosystem of the Earth serve to support and maintain life and allow it to recycle its waste!
Every mathematician knows how improbable it is for one lucky accident to be followed by another lucky accident, and then by another. By the laws of probability, the odds are not simply added, but multiplied. A very small probability multiplied by another small probability, then multiplied again by a very small probability, and so on, results in a probability that is practically nil. For the same reason, the likelihood of finding another planet, with the same favorable conditions as the Earth, is extremely low—so low that none has been found! There is a lot of excitement and speculation about some exoplanets discovered by the use of the Kepler Space Telescope – such as the planet identified as Kepler-452b – but there is no evidence or certainty that these planets support life.

Comparison of Kepler-452b Planet with Earth
Image source link: commons.wikimedia.org
A recent study determined that there are about 8 x 10^20 (or 800 quintillion) terrestrial planets (TP) in the universe. (See Zackrisson et al., Terrestrial Planets Across Space and Time, p. 5.) This number includes Earth-like and super-Earth planets in the size range from 0.5 to 10 times the Earth’s mass in the vicinity of FGKM stars in the Goldilocks zones of their respective galaxies. But these are not necessarily habitable planets, although they are in the “habitable” zones. Most of these planets (about 98% of them) are in M-dwarf host stars where X-rays, UV radiation, and flares from low-mass stars tend to erode the planet’s atmosphere and the prospect of liquid water. If only planets around solar stars (spectral type FGK) are considered, and the super-Earth planets are removed to ensure less gravity and make local motion manageable for large animals, the total number of TP candidates is reduced to just 2 x 10^18 (or 2 quintillion, per Zackrisson, p. 6). Although these exoplanets exist in the habitable zones of their galaxies, it remains to be seen whether they orbit their host star at the right distance; whether they have the right spin; whether they have water and the right ocean-to-continent ratio; whether they have the right surface temperature; whether they have the right atmosphere with the right kind of gases; etc. When all these criteria are applied, the number of prospective TP candidates quickly plummets toward zero. Thus, even the existence of Planet Earth appears to be a statistical anomaly in a universe ruled only by chance.
The Lucky Elements
The above discussion merely highlights the lucky conditions needed for a habitable planet. We also need to have large macromolecules that serve as the building blocks of life itself. The macromolecules needed for life are carbohydrates, lipids (fatty acids), proteins, and nucleic acids. Carbohydrates are the primary sources of energy in an organism; the lipids are the bases of cell membranes and act as a store of energy; the proteins, which are made up of hundreds to thousands of amino acids, comprise the tissues, parts, and organs of an organism and do most of the chemical processes in the body; the nucleic acids (DNA and RNA), which are found in the nucleus of cells, make the enzymes and contain the genetic codes to build the proteins.
Luckily, the universe has produced the elements with the right physical and chemical properties that could produce the building blocks of life. Some of these elements, such as hydrogen, were formed in the interiors of stars. The heavier elements were formed as the universe cooled. But the most fortunate thing is not that elements other than hydrogen were formed, but that some of these elements have precisely those properties that were needed to allow the formation of the macromolecules needed for life. In particular, the elements with special “lucky properties” are the following:
-
Carbon. Of all the elements found in nature, carbon has the unique property of attaching to itself in practically unlimited ways, thus allowing the formation of long chains of carbon atoms to which other elements can be joined. This unique property enables the production of the four important macromolecules needed for life.
-
Hydrogen. Its name contains the word “hydro” because it easily combines with oxygen to form water. Water itself is important to life because the cytoplasm of living cells is made up largely of water. But hydrogen is also important, not only because it combines with oxygen to form water, but because it combines with oxygen and carbon to make large carbohydrate and lipid molecules. It is also a component of large protein molecules and the complex structure of nucleic acids.
-
Oxygen. Like hydrogen and carbon, oxygen enters into the chemical structure of the four macromolecules needed for life. Additionally, it has a major role in cellular respiration. Cells use oxygen to make or release energy. On Earth humans and animals inhale oxygen from the air and exhale carbon dioxide as waste. Plants, on the other hand, absorb carbon dioxide from the atmosphere and release oxygen. How the Carbon-Oxygen Cycle works is a marvel of nature that is as lucky as it is awesome.
-
Nitrogen. This element is a component of the amino acids, which are the building blocks of proteins. Proteins comprise not only the muscles, tissues, and organs, but also the enzymes and hormones necessary for the various parts of the organism to function
-
Sulfur. This element is a minor constituent of some fats and body fluids but is a key component in certain amino acids, particularly cysteine and methionine. These amino acids have special cell functions in the body. Among other things, for example, cysteine plays a role in the communication between immune system cells.
-
Phosphorus. This element is essential for the creation of DNA and adenosine triphosphate (ATP), which is a high-energy molecule that stores and supplies the needed energy to living cells. That phosphorus has this energy-storing ability is pure luck. Many species of animals would not be able to live without phosphorus and its ability to store energy.

A Dipeptide formed by Two Amino Acids
Image source link: commons.wikimedia.org
Although water is not a macromolecule (because a molecule of water consists only of two atoms of hydrogen and one of oxygen), it has lucky properties that make it essential to life as well. Many biochemical reactions will not take place without water. In addition, it can dissolve a host of solid materials that need to be transported by the blood to the different parts of the body. Many compounds cannot dissolve food without damaging the tissues and organs of the body. But luckily, water has this ability, too.
It may not be obvious why the properties of the elements are crucial for a discussion on the chance origin of life. But in 1947, Prof. Lecomte du Nouy, in his book, Human Destiny, cited the calculations made by Prof. Charles-Eugène Guye to see how likely it would be to produce a single protein molecule necessary for life if those special properties were absent. To simplify the calculation, a hypothetical protein molecule is envisioned to consist of only two kinds of atoms. Actual protein molecules have at least four kinds of atoms – carbon, hydrogen, oxygen, and nitrogen, plus sulfur, phosphorus, copper, etc. In addition, it was assumed that the molecule has only 2000 atoms, with 1000 atoms of each kind. Actual protein molecules have considerably more atoms than this hypothetical molecule. Since proteins in living organisms are characterized by a certain asymmetry, a conservative value of 0.9 for dissymmetry was assumed. The two kinds of atoms are then represented by white and black balls that are to be placed at random inside a long glass tube. The question is then reduced to the following mathematical problem: What is the probability that, if the balls were placed in the tube at random, they would be arranged in such a way that 90% of the balls of each color would be on each half of the tube? The result is 2 chances in 10^321 trials, which is unimaginably small. (See Lecomte du Nouy, Human Destiny, p. 34.)
Of course, the chance of producing a molecule with 0.9 dissymmetry increases when there are more than just 2000 atoms available, since this will allow performing the experiment several times simultaneously. However, calculations by Prof. Charles Eugène-Guye indicate that the amount of matter necessary to produce a molecule with this probability would require a volume sextillion, sextillion, sextillion times greater than even that of the whole universe itself! Also, the chance of producing a molecule with 0.9 dissymmetry increases if there have been enough attempts (or trials) to produce the molecule. Prof. du Nouy reported that, assuming 500 shakings (or attempts) per second, which is of the same order of magnitude as light frequencies with wave lengths between 0.4 and 0.8 microns, the time that would be needed to produce a molecule of 0.9 dissymmetry is approximately 10^243 billion years. But life on Planet Earth appeared only 3.7 billion years ago! Thus, Prof. du Nouy noted that we are in the position of a player who does not have enough time at his disposal to throw the dice often enough to obtain the desired outcome. (See Human Destiny, pp. 34-35)
Of course, an event can still happen no matter how small the chance, but in that case, we could only expect one molecule, or at best two or three. But hundreds of millions of vastly more complex molecules are needed to make one living cell. This means that if everything were left to chance alone, then it would be practically impossible to produce life anywhere in the universe.
One might complain that the calculations made by Prof. Guye were unrealistic because actual atoms are not equivalent to the simple white and black balls assumed in the calculation. If actual atoms were used, the hypothetical molecule could be formed much more easily because actual atoms have properties that allow them to form large molecules easily, and the laws of nature further regulate the way they behave in nature. Ah, but that’s exactly the point! If everything was ruled by chance, then the properties of elements and the laws of nature would be as much a product of chance as the arrangement of the white and black balls. To assume a priori that the white and black balls have the special properties of, say, carbon and hydrogen, is to assume that the balls of the game are “loaded” and, therefore, the arrangement is not random.
So, why do these elements and compounds have precisely the kinds of properties that they need to have as if they were skillfully contrived to bring about the formation of the fundamental macromolecules necessary for life? Why does Mother Nature have laws that are preferential to the birth of life, if everything is really due to chance? What explains this anomalous preference, unless perhaps there is a “Cheater” in nature that is deliberately forcing the desired outcome?
The Most Lucky Event: The Formation of the First Living Cell
It is indeed lucky that as the universe developed and as the stars cooled, elements with the right properties necessary for the formation of organic molecules were also produced. It is also very lucky that there is a Planet Earth that has all the physical characteristics needed not only to begin life but also to sustain it. However, merely having the right conditions on Earth and the presence of the right elements is not enough for the formation of the first living cells, as scientists now realize.
In 1952, two biochemists, Stanley Miller and Harold Urey, conducted an experiment in which they tried to simulate the conditions that were presumed to exist on Earth before life began. They set up an apparatus that held a mixture of water vapor, methane, ammonia, and hydrogen – gases similar to those thought to comprise the Earth’s early atmosphere over a pool of water, which represents the Earth’s ocean. Electric current was introduced into the gas chamber to simulate lightning. The process was permitted to run for a week. When they examined the contents of the liquid chamber, they found several amino acids that collected together in the form of coacervates. Among the acids formed were glutamic acid, aspartic acid, alanine, and glycine.

There was much rejoicing following the successful production of these organic compounds. But what needs to be pointed out, and what no one is talking about, is that the right combination of gases, the temperature of the water, and the electric spark were all deliberately introduced into the apparatus. Therefore, the organic compounds obtained from the experiment were not produced purely by chance.
Also, the problem that scientists see today is that the actual atmosphere of the early Earth might not be the ammonia-rich, hydrogen-filled atmosphere that they had used in the laboratory. Recent research has revealed that the early Earth’s atmosphere was more oxidizing than reducing, with more oxygen, carbon dioxide, and sulfur dioxide than hydrogen, methane, and ammonia. See NASA Research Highlight, Earth’s Early Atmosphere: An Update. Under these conditions, the amino acids and nucleotides needed for life are considerably more difficult to synthesize, for any organic compound produced would have been immediately broken down by the free oxygen in the atmosphere.
Anyway, the Miller-Urey Experiment was performed, but no living cell was produced. In the years following the Miller-Urey experiment, several other experiments were attempted in the hope of producing the first synthetic living cell. But to this date, no living cell has been produced.
Scientists are now beginning to realize that abiogenesis—or the formation of life from inorganic matter—is a process vastly more complicated than they had anticipated. One reason the Miller-Urey experiment failed to produce a living cell was that the prebiotic molecules produced in the laboratory were a random mixture of right- and left-handed acid molecules, whereas the amino acids observed in living organisms are all left-handed ones. At that time there was no known artificial process of producing amino acids comprised only of left-handed molecules. Only living organisms were known to produce them because they had enzymes that selected the left-handed molecules and even converted right-handed molecules to left-handed ones. Amino acids need enzymes to produce proteins, but enzymes were absent in the coacervates. Enzymes were produced exclusively by secretion from living cells, and precisely there was no living cell yet.
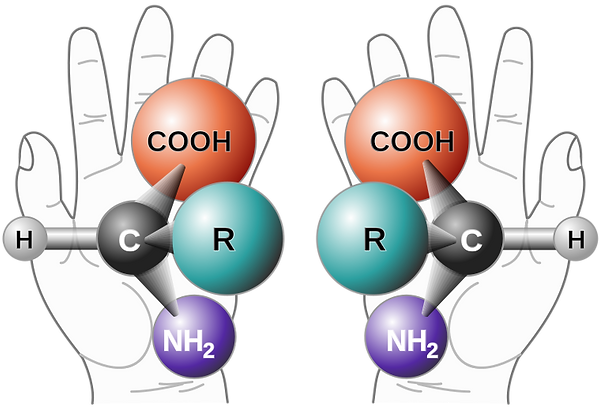
Left- and Right-Handed Amino Acid Molecules
Image Source Link: commons.wikimedia.org
Making a synthetic living cell proved to be a difficult task for molecular biologists. In the laboratory, modern molecular biologists have been able to synthesize viruses, which are primarily made up of nucleic acids (DNA or RNA) encased in a protein coat, but they have not been able to create one-celled creatures like bacteria from scratch. Like bacteria, viruses have the genetic code to replicate. But unlike bacteria, viruses do not have or make their own enzymes. They need to borrow enzymes from a living organism to show any sign of chemical activity. Outside an organism, viruses are practically inert. They need the enzymes of living organisms to thrive and replicate.
In 2010, J. Craig Venter and his companions were able to copy, modify, and synthesize the DNA of the M. mycoides bacterium and transfer it into the cell of a closely related bacterium, M. capricolum. Despite the success of Venter's team in making synthetic DNA, one cannot say that they started from scratch. First, they did not make a new cell but used an existing cell as a home for the new DNA. Second, they did not write a new code for the synthetic DNA but merely copied the existing DNA code of the M. mycoides bacterium. The only modifications they made to the DNA were to add base strings that serve as “markers,” so that they could distinguish the synthetic DNA from the natural one. Writing a new DNA code is not that easy, for it has to be compatible with the existing structure of the entire organism. When the synthetic DNA was first installed into the M. capricolum, it didn’t work right away because of just one base mistake in the synthetic DNA, indicating how precise the code has to be for the DNA to work properly. See Creation of a Bacterial Cell...
There is a very important reason why synthesizing a living cell from scratch is extremely difficult. This can be seen by observing the big difference between the viruses produced in the laboratory and the simplest living bacteria observed under a microscope. Viruses have only one kind of nucleic acid, whereas even a one-celled bacterium has different kinds of nucleic acids, each with its specific function. Some enzymes fabricate the cell wall, others enable nucleic acids to reproduce or duplicate themselves, and still others manufacture and sustain proteins that comprise the parts of the cell protoplasm. A living cell must have all these components: proteins, nucleic acids, enzymes, and a cell wall to exist. These components work together as a unit and are interdependent. Proteins cannot be made without the right DNA code from nucleic acids. The nucleic acids in turn need the proteins to regulate the activity of the cell according to the code. Nucleic acids (DNA) are needed to make enzymes, but enzymes are needed to make nucleic acids. Cell walls are made by enzymes, and the enzymes in turn need the protection of the cell walls to do their job. The cell wall filters the materials coming into the cell, absorbing only what is needed by the proteins, the nucleic acids, and the enzymes, while keeping the vital enzymes from escaping. Since the components of the cell are interdependent, none of them can be produced ahead of the others. This is why it is such a big challenge for scientists to produce even one living cell from dead matter. All components must already exist and function simultaneously to make a living cell, but they cannot exist or function simultaneously unless they are already working parts of the cell that they are supposed to produce. That is the problem.
Isn’t it a most fortunate event then that in nature, all components of a cell happen to be present at the right time and at the right place to produce the living cell? Incidentally, each of these components is also a very complex biological structure in itself, with characteristics and functions that benefit the whole organism. Each component’s structure and function are meaningful only as parts of the organism; that is, these components are useless in and of themselves, and their sole purpose is to serve the organism and keep it alive. The question then is, if life evolved from non-living matter, how did these elements even begin to exist and do the function of serving the needs of the living cell before there was any living cell to serve? Scientists don’t have a clue.
So, did life originate from non-living matter purely by chance? The obvious answer is, "No, it most likely did not."
Then how did life originate on Earth? Without going deep into philosophy or theology, the short answer—which many scientists do not like to hear—is God. It was God Who invented the laws of nature, the “Cheater” Who loaded the atoms of various elements with properties preferential to the birth of life. It was God Who directed the physical and chemical changes that led to the formation of the first living cell, and it was His Intelligence that wrote the genetic code that directed the evolution of wondrous organisms on Earth. “Oh, no!” a scientist might object. “We cannot trace the origin of life to God because His existence and the statements made about Him cannot be empirically and perceptually verified.” To which we may reply, “Correct, dear scientist. The existence of God and His activities in the world are not perceived by the senses; they are grasped by the mind.” Science can only describe the observable things that living cells do, but it has no knowledge of the ultimate cause of living cells or the purposes behind their marvelous behavior. These hidden causes are for the mind of man to discover. Nor is science interested in the beauty of nature. It may be able to describe how the colors of the rainbow are produced, but it cannot explain the beauty that the rainbow evokes in the heart of someone who beholds it. That is outside its scope of inquiry. The investigation of metaphysical truth, beauty, and the ultimate cause of being is the work of the philosopher, not the scientist. And philosophy begins where the sciences end.
Q & A
1. What makes protein molecules and nucleic acids difficult to synthesize?
The proteins and the nucleic acids (DNA and RNA) needed to produce life are very intricate structures in themselves, with parts that need to be precisely sequenced or placed to work. Consider the following:
-
Protein molecules are made from twenty distinct amino acids in left-handed configurations. These amino acids have to be sequenced in a particular manner and to a specified length to be useful. Thus, the acids need to be bonded first into short chains, then the short lengths need to be bonded again into longer chains until the right length is achieved; finally, these chains need to be sequenced one after the other in the proper order, or else this long chain of amino acids, called a polypeptide, won't function the way it is supposed to work in the organism. The chains of a protein molecule can wind and loop around, as the picture below shows. Changing an amino acid in the chain or altering the sequence of amino acids in the chain will alter the physical characteristics of the polypeptide and could result in an entirely different protein or biologically useless molecule. Now, what controls the sequence of amino acids that produce the different kinds of proteins found in a living cell? It is the genetic code in the DNA that will be discussed next.

3D Structure of the Protein Myoglobin
Image source link: commons.wikimedia.org

3D Structure of DNA
Image source link: commons.wikimedia.org
-
Nucleic acids (DNA and RNA) are even more complex than protein molecules. The DNA (short for deoxyribonucleic acid), which is the main constituent of chromosomes in the nucleus of a cell, is less plentiful than the proteins, but it contains the genetic code or "blueprint" that controls the building and function of the proteins in the various parts of the organism. Unlike protein molecules, which are long chains comprised of twenty different amino acids, the DNA molecule is comprised of only four types of nucleotides grouped by two strands of sugar-phosphate chains that twist around like a double helix, forming a polynucleotide chain. The four nucleotides are adenine (A), thymine (T), guanine (G), and cytosine (C). The nucleotides cytosine and thymine are formed by a single ring and are called pyrimidines; adenine and guanine are formed by a double ring and are called purines. Just as the sequence of amino acids determines the specificity of a protein molecule, so the arrangement of the four different nucleotides determines the genetic code in a DNA strand. The sequence determines how new proteins and enzymes will be built, how they will function, how the organism will reproduce and whether the entire organism will grow into an orchid or a ladybug.

-
RNA (short for ribonucleic acid) is similar, but usually (though not always) consists of a single strand of nucleotides that folds upon itself rather than a double strand. An RNA’s nucleotides are adenine (A), uracil (U), guanine (G), and cytosine (C), and it contains sugar ribose rather than deoxyribose. The sugar ribose differs from the deoxyribose by the presence of an additional -OH group. During protein synthesis, the instructions contained in the DNA are first transcribed or copied into an RNA, and then the RNA works as a messenger that carries the instructions from the DNA to control the construction of the proteins. There is evidence that the RNA itself also sometimes works as an enzyme that controls the production of the protein.

This RNA sequence shows how triplets of nucleic acids translate into amino acids
Schematic illustration by Thomas Splettstoesser
CC BY-SA 4.0 license: commons.wikimedia.org
2. Would the presence of DNAs and RNAs in the laboratory easily lead to the fortuitous production of a living cell?
No, not easily. Merely having the parts of a cell does not guarantee that a living cell will be produced by chance. A mechanic can go to a large junkyard and find all sorts of parts to build a car. But he wouldn't know which parts were suitable and how they would all fit together in the entire assembly. He needs the engineer's plan or blueprints to assemble a car. He needs to find the right part that was built to the right specification for the car model that he wants to assemble. A mismatched bolt or misaligned hole will not work. Assembling the DNA sequence from scratch and building the necessary components for a living cell are a thousand times more complex than making a car at a junkyard. Even today, biochemists have no clue how the genetic code sequence (the "blueprint") that links the nucleic acids to amino acids originated in the first place. The genetic code is like a software code that determines how tissues will be formed, how each part of the organism will function, and what kind of organism will be produced. The beautiful designs on the wings of a butterfly are determined by the code. They did not "just happen." The question is how the code for each wondrous organism in nature got there. Finding the answer to this question by science alone is not easy, and scientists are having a hard time.